Types of Medical-Grade Rubber Seals
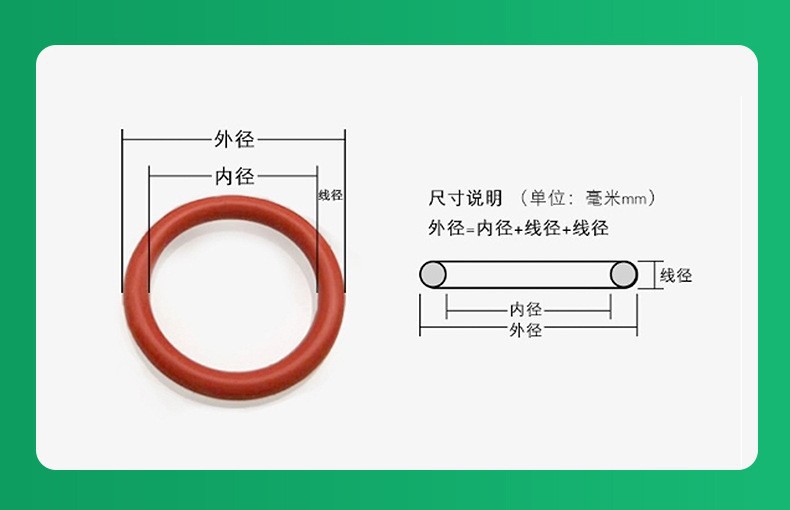
- O-Rings
- Features: Circular cross-section, high elasticity, and compression resistance.
- Applications: IV pumps, syringe connectors, and surgical instrument housings.
- Gaskets & Diaphragms
- Features: Flat or molded designs, chemical-resistant, and pressure-tolerant.
- Applications: Drug delivery systems, ventilator valves, and diagnostic equipment.
- Septum Seals
- Features: Self-sealing puncturable rubber for needle access.
- Applications: Vaccine vials, IV bags, and blood collection tubes.
- Gland Seals
- Features: Reinforced structures for dynamic motion.
- Applications: Rotary shafts in dialysis machines and imaging devices.
- Custom Molded Seals
- Features: Patient-specific geometries (e.g., prosthetics, implantable devices).
Key Characteristics
✔ Biocompatibility – Safe for prolonged contact with tissues/fluids (ISO 10993 certified).
✔ Sterilization Resistance – Withstands autoclaving, gamma radiation, and EtO treatment.
✔ Chemical Resistance – Immune to drugs, alcohols, and aggressive disinfectants.
✔ Temperature Range – Stable performance from -60°C to +250°C (-76°F to +482°F).
✔ Low Particulate Shedding – Critical for cleanroom and pharmaceutical use.
Industry Applications
- Medical Devices: Infusion pumps, ventilators, endoscopes.
- Pharma Packaging: Sterile vial stoppers, inhaler valves.
- Diagnostics: Lab analyzer seals, centrifuge lids.
- Implants: Silicone seals for pacemakers, insulin pumps.
Safety & Compliance
Our seals comply with:
✅ USP Class VI – Rigorous testing for plastics/rubbers in medical use.
✅ FDA 21 CFR 177.2600 – FDA-approved elastomers for food/medical contact.
✅ EU MDR 2017/745 – Meets EU Medical Device Regulation requirements.
✅ ISO 13485 – Quality management for medical device manufacturing.
Customization Services
We engineer seals to your exact needs:
- Material Selection: Silicone, EPDM, FKM (Viton®), or Nitrile (NBR).
- Precision Molding: Micro-tolerance seals down to ±0.005mm.
- Post-Processing: Plasma treatment for enhanced adhesion.
- Validation Support: Full documentation for FDA/CE submissions.
Maintenance & Care
- Cleaning: Use isopropyl alcohol (IPA) or mild detergents; avoid abrasive cleaners.
- Inspection: Check for cracks, swelling, or compression set monthly.
- Storage: Keep in anti-static bags at 15–25°C (59–77°F), away from ozone/UV.
- Replacement: Follow OEM lifecycle guidelines (typically 3–5 years for implants).
Why Choose Our Medical Seals?
- Zero Defect Guarantee: 100% inspected for critical applications.
- R&D Partnerships: Co-develop seals for novel medical technologies.
- Fast Prototyping: 3D-printed samples in 72 hours.
Contact us to secure FDA-compliant seals for your next medical innovation! 🚑🔬
(Word count: 398 | Keywords: medical rubber seals, USP Class VI, FDA-compliant, custom O-rings, ISO 10993)